The selection of pots and pans can be a complicated affair. The shape of the cooking surface and handle(s), materials used in its construction, the intended purpose of the utensil's design, and its flexibility of use in the kitchen all are important factors in choosing cookware. Understanding the materials used is a good first step in understanding how cookware works and what factors may be important to your cooking style.
Related Articles
The purpose of cookware is to impart energy to ingredients. In America, the energy comes mainly in two forms: burning natural gas or propane gas and electrical resistivity. In both methods, the source of the heat is not uniformly spread over the pan. In a gas stove, the gas come out at regular intervals and forms a ring of individual flames. The heating elements of an electric range are designed to cover as much area as possible, but still have patterns (usually spirals) where there is no heat. Because the heat is not applied evenly, the cook must be aware of this and either compensate with cooking technique or through cookware.
High quality cookware should not only be durable, but also take the energy from the heat source and effectively transmit this energy to the ingredients. There are several factors that affect this capability. The two most important factors are thermal conductivity and heat capacity. Almost all discussions concerning the materials used in cookware are focused on these two factors.
Thermal conductivity
In short, the thermal conductivity of a material is how readily that material absorbs and transmits (releases) energy. When the fire or heating element of a range comes in contact to a pan, the energy from the heat source is transmitted to the pan. This increases the internal kinetic energy of the pan (commonly called "heating up"). The heated material then transmits the energy to nearby materials that are at a lower average molecular kinetic energy level (at a lower temperature than the material). The higher the thermal conductivity of the material, the faster it will heat up and also, the faster the heated area will spread to unheated areas of the same piece of material.
For example, if we placed a large sheet of stainless steel (fairly low thermal conductivity as cooking materials go) on a burner and turned on the burner, the area directly under the burner would get hot while the rest of the sheet slowly heats up. The burner imparts heat quickly only to the region of steel directly over it. The rest of the pan heats up from the conduction of the heat from that spot. When the outer edges of the sheet have reached a hot temperature, the spot directly over the burner would be extremely hot. The figure below shows an example of the temperature of the sheet of steel over a gas burner. The hottest parts are shown in white, hot is red and cool is blue.
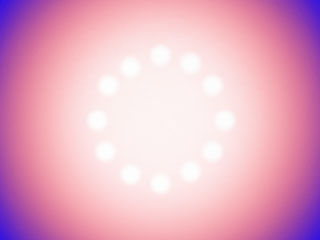
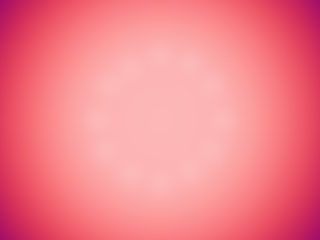
One solution to this problem is to make the sheet thicker. When heating a thick piece of steel (instead of a thin sheet), the bottom surface of the steel does not have the same temperature pattern as the top surface. Because the top surface is a greater distance from the heating element, the energy needs to conduct from the bottom to the top (just like the energy conducts outwards). The top surface of the steel is more evenly heated in this case. The figure below shows the thick sheet of steel after it has been sliced so the center of the front edge is where the burner heat touches the bottom of the sheet. The hot spot (white) is reduced by the time the heat conducts to the top surface of the sheet. Where the sheet is being heated, the temperature is more uniform now, but we still have uneven heating with this material.

For this reason, the thicker the steel, the less variation in temperature on the top surface. Unfortunately, low thermal conductivity means it a lot of energy needs to be imparted to the bottom of the steel in order to get the top hot. So a pan made of a low thermally conductive material will take a longer time to reach cooking temperatures. In fact, materials with low thermal conductivity take longer to react to any change in temperature, so the thermal response of the pan would also be slow. (Thermal response is how quickly the surface temperature of the pan reacts to when we increase or decrease the flame of the burner.)
In most cooking applications, it is desirable to have the utensil heat up quickly, not develop hot spots, and react to changes we make to the range controls. Materials with high thermal conductivity fulfill our needs because they transmit heat quickly resulting in fast response to thermal changes and even distribution of the internal kinetic energy.
Here is a list of some common materials used in cookware and their respective thermal conductivity:
| Material | Thermal conductivity |
|---|---|
| Copper | 401 W/m*K |
| Aluminum | 237 W/m*K |
| Cast Iron | 80 W/m*K |
| Carbon steel | 51 W/m*K |
| Stainless steel | 16 W/m*K |
Heat capacity
The amount of internal kinetic energy stored in a material can be referred to as it's heat capacity. This isn't the same thing as temperature, which is the average molecular kinetic energy within the material. For example, a kg of water at 100°F contains more energy than a kg of steel at 100°F. While thermal conductivity describes the materials ability to absorb energy, heat capacity is the amount of energy that is needed to raise or lower the temperature of the material. The molecular composition of some materials is such that as they absorb energy, much of it gets converted into potential energy and only a small amount increases the molecular kinetic energy (water is a common example). Other materials, like most metals, increase their molecular kinetic energy readily and do not store much of the absorbed energy as potential energy. The heat capacity of a material is proportional to its mass. So, a 2 kg piece of steel has double the heat capacity of a 1 kg piece of steel (make sense, right?).
What this means is that cookware made of materials with high heat capacity, will take longer to heat up, but will also have a significant amount of energy stored up when it is hot. When energy is pulled out of the material, the temperature of the material will lower slowly when compared to materials with low heat capacity. Cast iron is often cited as an example of a high heat capacity cookware material. The specific heat (the heat capacity of a material for a given mass) of cast iron is half of aluminum's specific heat, but because cast iron cookware is generally several times the mass of aluminum cookware, it has a much higher heat capacity.
The thickness of metals used in the construction of cookware are often sited by the manufacturer (for example, 3 mm aluminum), but since heat capacity is a function of the mass of the material, density must be known to make comparisons between cookware of different materials.
| Material | Specific Heat | Density |
|---|---|---|
| Aluminum | 910 J/kg*K | 2600 kg/m3 |
| Stainless Steel | 500 J/kg*K | 7500 - 8000 kg/m3 |
| Carbon Steel | 500 J/kg*K | 7500 - 8000 kg/m3 |
| Cast Iron | 460 J/kg*K | 7900 kg/m3 |
| Copper | 390 J/kg*K | 8900 kg/m3 |
Looking at the table above, if you multiply specific heat with density, you'll find that the heat capacity per unit volume of steel, cast iron, and copper are about 1.5 times that of aluminum. This means, to achieve the same heat capacity in an aluminum pan as in stainless steel pan, the aluminum pan needs to be 1.5 times as thick (assuming the other pan dimensions are the same).
Pulling it together: thermal diffusivity
If you've been paying attention, you'll realize that I've misled you when I discussed thermal conductivity. Thermal conductivity alone does not determine how fast the pan will heat up (and also how evenly it will heat). In fact, the heat capacity plays a role in determining this as well. Wouldn't it be great if we had a single number that told us at what rate heat would transfer through and spread out in the material? There is, it's called the thermal diffusivity of a material and is simply the thermal conductivity divided by the unit heat capacity (specific heat times density). Let's take a look at how the materials stack up:
| Material | Thermal diffusivity |
|---|---|
| Copper | 120 * 10-6 m2/s |
| Aluminum | 100 * 10-6 m2/s |
| Cast Iron | 22 * 10-6 m2/s |
| Carbon Steel | 14 * 10-6 m2/s |
| Stainless Steel | 4.3 * 10-6 m2/s |
Without additional calculations based on the heat conduction equation, there is very little that we can do with this table of values, except compare the materials against each other. It is clear, however, that the best performing materials (in terms of dishing out energy) are copper and aluminum. This leads us to our final consideration: reactivity.
Reactivity
Not only do we have to concern ourselves with the thermal properties of materials, but we need to make sure that the materials we use in our cookware do not harm us or adversely affect the taste of our food (you decide which is worse). For this reason, in addition to the high thermal diffusivity, we would also like a non-reactive material. Unfortunately, both copper and aluminum react readily to foods. (Copper, when ingested in quantity or consistently, can cause liver, stomach, and kidney problems as well as anemia. Also, aluminum has long been suspected of contributing to Alzheimer's disease. Oh, every cookbook mentions, at this point in the discussion, that the occasional foamed egg white whipped in a copper bowl is not enough to harm you - but refrain from cooking every day on exposed copper.) Stainless steel, the least reactive of all popular materials used in cookware, also has the worst thermal diffusivity.
It seems that today, physics is not our friend. But, through the magic of cookware companies wanting to find ways to charge us lots of money, solutions have been devised to enable us to enjoy cookware made of materials with high thermal diffusivity and low reactivity. By combining the non-reactive surface of stainless steel with the thermal properties of copper or aluminum, you get the best of both worlds. There are several variations on this theme: steel- or tin-lined copper, stainless steel with aluminum or copper disk, stainless steel cladded aluminum, and stainless steel cladded copper. The table below summarizes my subjective assessment of the effectiveness of various material combinations (they are listed in order from most effective to least):
| Rank | Composition | Comments |
|---|---|---|
| 1 | Copper with tin lining | Highest response; tin lining can be finicky can be susceptible to melting; copper exterior requires more care |
| 2 | Copper with stainless steel lining | Copper exterior requires more care but imparts the utensil with copper's excellent thermal properties |
| 3 | Aluminum with stainless steel lining | Thick aluminum provides excellent thermal response to thin steel interior |
| 4 | Copper fully clad by stainless steel | Copper layer may be thinner than copper with stainless steel lining; exterior and interior are durable and easy to maintain |
| Aluminum fully clad by stainless steel | Aluminum layer may be thinner than aluminum with stainless steel lining; exterior and interior are durable and easy to maintain | |
| Aluminum with stainless steel lining and copper exterior | Same performance as cladded aluminum, but with the difficulties in maintaining copper | |
| 5 | Stainless steel with copper disk | Curved edge of the bottom causes the disk to not come into full contact with the complete bottom of the pan resulting in inferior heat conduction as compared to cladded copper |
| Stainless steel with aluminum disk | Same as stainless steel with copper disk |
Previously, I mentioned that cast iron has a large heat capacity as compared to the other materials (mostly because of the mass used when making the cookware). Because of this attribute, cast iron gets a special place in the kitchen. When the cooking task requires the ability to maintain consistent heat (and ample amounts of it), nothing beats cast iron. Because cast iron can react with acidic foods and ingredients that are cooked for a long time, cast iron cookware is seasoned - a process by which layers of fat are slowly cooked into the porous iron until the fat polymerizes forming a protective barrier (and makes the utensil relatively non-stick).
Common materials and how they compare
Now that we've looked at the important properties in selecting cookware material, let's take a look at each of the common materials used in cookware.
| Copper | |
|---|---|
| Description | Copper is a soft (scratches easily) but durable (will last a lifetime) material that has great thermal properties. The material is prone to oxidation but with care, will retain its beauty indefinitely. |
| Pros |
|
| Cons |
|
| Best uses |
|
| Care |
|
| Examples |
|
| Aluminum | |
|---|---|
| Description | Plain aluminum utensils are low-cost, light-weight, and thermally responsive - but it's reactive. Teflon coated aluminum utensils are low-cost and both non-stick & non-reactive. Anondized aluminum has been treated to develop an aluminum oxide (extremely hard and non-reactive) coating on the surface of the utensil. Clad or lined aluminum has had stainless steel bonded to the interior of the utensil to form a non-reactive surface. |
| Pros |
|
| Cons |
|
| Best uses |
|
| Care | Hand-wash with a mild detergent and washcloth or sponge. |
| Examples |
|
| Cast iron | |
|---|---|
| Description | Cast iron is composed on iron, carbon (more than carbon steel), and trace elements found in common clays. The iron is melted down and poured into a sand or clay mold to form the utensil. Enameled cast iron has a thin but durable nonreactive layer of glass fused to the surface of the utensil. |
| Pros |
|
| Cons |
|
| Best uses |
|
| Care | Plain cast iron should be seasoned before first use and as needed. A seasoned utensil should receive minimal contact to soap or detergent. Wash by soaking in warm water for a few minutes and repeatedly scrubbing with salt and rinsing until salt remains white (usually one scrubbing is does it). Dry with a cloth and heat over low heat briefly to evaporate all moisture. For enameled cast iron, hand wash in hot soapy water. |
| Examples |
|
| Carbon steel | |
|---|---|
| Description | Carbon steel contains less carbon than cast iron and is formed and pressed from sheets instead of being casted. It can be annealed (heating the metal until its molecular structure realigns to alleviate internal stresses and then specially cooled to preserve the new structure) to form blue steel (or black steel), a harder and less reactive material. Carbon steel can also be enamel coated. |
| Pros |
|
| Cons |
|
| Best uses |
|
| Care | Should be seasoned before first use. Care for as if it was cast iron. If desired, pan can be washed in soapy water, scoured, and reseasoned quickly (15 minute seasoning) because of its less porous nature than cast iron. |
| Examples |
|
| Stainless steel | |
|---|---|
| Description | Mixing steel with chromium and nickel (18/8 stainless steel is 18% chromium and 8% nickel while 18/10 has 10% nickel) produces a corrosion resistance steel that is both hard and easy to maintain a shine. Disks of copper or aluminum can be fused to the stainless steel cookware to enhance its thermal properties. Stainless steel can also be used to line copper or aluminum utensils as well as cladding aluminum or copper (see aluminum and copper cookware summaries above). |
| Pros |
|
| Cons |
|
| Best uses |
|
| Care | Hand wash with mild detergent. Use gentle abrasives as needed. |
| Examples |
|
My personal favorites for cookware materials are stainless steel clad aluminum or copper and cast iron (for skillets and woks). The stainless steel clad utensils perform well, are easy to clean, and look beautiful. Of course, not all stainless steel clad aluminum (sometimes called tri-ply or five-ply depending on construction) are the same. All-Clad has definitely earned their reputation as quite possibly the best general use cookware money can buy, but it's a lot of money to be spending. All-Clad rarely goes on sale, but other reputable brands, such as Calphalon, have clad lines as well - and they are more likely to have their product lines go on sale. Keep checking the Cooking For Engineers Deals Blog to see when deals do come up.
Related Articles

Excellent post. I've been wondering recently whether to upgrade my cookware... and to what. I'm using a Viking saute pan at the moment, which i believe is very thick stainless steel with an aluminium base... but i find it takes so long to heat up that i often prefer cooking with my cheap K-mart brand frying pan...
After reading your post, it's confirmed a number of thought's i've had... in regards to heat transfer and capacity. I'm thinking a nice Mauviel saute pan would look great on my stove top.
Cheers :)
Matt
I was hoping to see something about Teflon (Or any kind of non-stick coating), as well.. How it works, care it requires, etc. Will you write something about it in the future?
If you live in a metropolitan area you can often find them at garage sales -- and at 50 cents to a couple bucks per pan, this excellent cookware is a steal.
Often you can find the older stuff on eBay, as well. I recently puchased a huge skilllet, with lid, of this vintage Farberware for about 15.00.
In the future, I will have a (shorter) discussion on non-stick cookware. My current problem is that I can't seem to get enough info on the newer non-stick solutions (Scanpan, etc.) and I'd like to include those as well.
I have a few cybernox pans, and they're great, but I have no idea what they actually are.
Of course, I don't really like the taste of aluminum in my foods...
However, many of the non-stick coatings outgas irritating or toxic gases when heated strongly (which those of us who stir-fry a lot tend to do). These gases can sicken people and kill birds.
Teflon is a persistent chemical.
Are these types of pans still available?
How does the cookware feel when you handle it? Does the handle get Hot? Will it take hours a week to keep clean/looking nice? The easiest way to answer the question is to recommend any verison of the "All Clad" cooking tools. They come in both regular and nonestick, handles stay cool, they are comfortable, conduct heat well and evenly considering the Stainless Steel quotient, and will be the last set of pots & Pans you'll ever buy. Oh yea, "all Clad" is always rated in the top two by Consumer Reports/Cook's Illustrated etc., not to mention most major cooks without a cookware deal use it also, just watch the food network/TV.
Don't get me wrong, you can go with Faberware, and TFal and so on and so on, but you'll replace it every 5 to 7 years, and eventually pay more in a lifetime than if you bought "All Clad" up front.
Check out the egullet understanding stovetop cookware for another excellent take on this topic.
Cookware Safety
FDA article
Might be a bit out of date, but still handy
inch casserole pan from Nordic Ware. It has
badly applied non stick that I am pleased to see
is rapidly flaking off. It is steel so will
work on my induction cook top. The greatest
use is for baking between the smooth hot
upper and lower plates of my Griddler panini griddle.
It makes beautiful casseroles, bakes a roast, or heats frozen entres.
If I need a lid
another pan inverted and held on by two
one inch paper clamps that squeeze easily around
the two rims.
It has many uses except for microwaving.
This type of cookware is thin aluminium coated with enamel inside and out. Used by our Grandmothers when cooking and still around. Relatively cheap. Usually found in camping stores or where you find home canning supplies for the larger pots.
Excellent if you want to boil anything. Horrible if you want to cook something slowly.
Will there be another article on what works best in an oven?
The worst cases have required some soaking with water and dish detergent but that's it. And there's no seasoning required, unlike cast iron.
The only thing that really stuck to the All-Clad was a melted $3 plastic steam basket. (don't ask)
Freezing, WD-40, Simple Green and Wright's Silver Cream cleaned the pan up nicely. My only hope is that I didn't damage the corrosion resistance of the stainless steel.
Does anyone know if I could have permanently damaged the stainless steel somehow?
Here's a fun place to start inquiy into stainless:
http://www.corrosion-doctors.org/MatSelect/corrstainsteel.htm
I had read about the chromium-oxide "layer" on stainless steel (see below) and wondered if I had damaged the corrosion resistance of the All-Clad, or any of its inherent non-stick properties.
I'm really out of my element here so my questions might seem a bit "daft." ;)
Is the chromium-oxide layer important for cookware? If so, does it require maintenance or special care?
Thanks.
"Stainless steel can corrode in service if there is contamination of the surface. Both pickling and passivation are chemical treatments applied to the surface of stainless steel to remove contaminants and assist the formation of a continuous chromium-oxide, passive film."
"The purpose of passivation of the surface is not only to clean and remove free iron, but to maximize the chromium content of that top, very thin "layer" of chromium oxide. (Other metals in the alloy also greatly affect this.) This gives the best corrosion resistance,"
"As you are no doubt aware, one of the attributes of stainless steel is the fact it has a built in oxide layer over it. This is in the form of chromium oxide and it is this that gives it its corrosion resistance. If you want to enhance this, you have to be careful how you do it; if you simply anodically polarise it, you will run the risk of breaking down the existing layer and dissolving out the metals. You could try putting it in hydrogen peroxide and leaving it, or better still, try electropolishing it."
geo
Regarding the possible health risks of using teflon, there are none.
http://www.cei.org/gencon/019,04820.cfm
It is true that there may be some health risks caused by a chemical used to <b>make</B> teflon (namely perfluorooctanoic acid, or PFOA), but that is a problem for chemical factory workers and perhaps people who live near the factories, not people who use teflon. There is no PFOA in actual teflon.
http://www.planetark.com/dailynewsstory.cfm/newsid/29003/story.htm
Teflon itself is chemically unreactive; in fact, it is one of the least reactive substances ever discovered. If we ate some teflon, it would pass through our bodies unchanged. None of it would enter our blood stream. But even if it did, it would would not affect any of our biochemical processes, since it is unreactive. It would interact with the body about as much as a stone reacts to me shouting at it. In fact, that unreactivity is part of why it is so slippery. Maybe the subject of another article in Cooking for Engineers?
People pay lots of attention to health scares, as they should, but they often don't take time to look at it carefully. PFOA is not an "ingredient" of teflon, it is one of the chemical precursors of teflon, like oil is to polyurethane. If oil is bad for you, it doesn't mean that polyurethane is. (And in fact, high-density polyurethane is totally inert in the body just like teflon, so it is used in artificial joints and the like.)
Like EMF radiation from powerlines, this one appears to have been cooked up by trial attorneys looking to make a buck.
Karl
http://news.bbc.co.uk/2/hi/science/nature/3441255.stm
Admittedly, canaries are not humans, but Teflon seems to break down under high temperatures according to one group of researchers (absolutely not! says the other side - Teflon is unreactive).
It's always like this - everyone argues and there are strong motives for lying and exaggeration - but at the end of the day we're built out of the same basic stuff as canaries so I think I will give Teflon a miss - if it's killing them then it's most likely partially killing me...
(from the viewpoint of an economist in his 20s) < always important for evaluating info i.m.o - how much do they really know about x, y or z after all... perhaps not too much in my case, but I do like to think critically - I dislike selective evidence - now you have both sides to consider. ^.^
[edit] from that link posted earlier:
Although nonstick pans will wear away with hard use and particles may chip off, the Food and Drug Administration has stated that these particles would pass unchanged through your body and pose no health hazard. A coated pan heated for long periods at high temperatures will give off fumes, but these are less toxic than fumes given off by ordinary cooking oils.
Do these people ordinarily cook with crude oil or something!??!
Runner up is stainless clad aluminum, but it is harder to clean and not as nonstick, and much more expensive.
To add a data point:
After much research and reading here and else where, I picked up the member's mark tri-ply cookware at Sam's club. I could never afford an All-clad set right off so this get's me the same performance at a great price. So far the quality and performance is right up there with All-Clad. I know I'll probably pop for something from the big "A" just for the fun of it but I'm very happy with my selection.
Reading about material and construction/performance here was a great help in my selection.
THanks!
I am very close to picking up a set of Copper-clad cookware from All-clad. It is copper-lined w/ stainless steel coating so it should have the best of both worlds- good heat properties and low reactivity with food. But, it will not work as good with an induction cooktop as good, old-fashioned steel or cast-iron.
There are several things I like about induction cooktops (stays cool to touch), but the biggest is it's rated efficiency. Can anyone compare the efficiency of an induction cooktop to a high-end ceramic cooktop? What about the heat properties of a good stainless steel set on an induction stove to a aluminum/copper clad set on a decent stove?
~Thanks
The oxide layer on stainless steels is (a) self-maintaining, and (b) very diffficult to remove. Unless you're performing electrochemical experiments in your cookware, or cooking with concentrated acids, you won't damage the stainless. If you are doing those things, the condition of your cookware is probably not your biggest problem.
What makes stainless steel so much nicer than regular steels is that chromium oxide is not air-permeable, so only a very thin layer at the surface reacts. Iron-oxide, which developed when normal steel reacts in air, is much less attractive and is air permeable. As a result, a piece of mild steel can corrode all the way through.
I was wondering a while back, while thinking of excuses not to clean the copper bottom of my cheap stainless steel pan, whether the better radiative heat transfer that I'm bound to get from the non-shiny, more darkly colored surface of my discolored copper is worth decrease in conductivity that I assume I will get due to the oxide layer that is causing the discoloration.
Obviosly this is assuming that I'm not concerned with the asthetic aspects of the pan, since shiny things are definitely prettier.
Anybody happen to know?
As far as the Alzheimers thing goes. Studies have shown that the original premise was false. Doctors had been finding elevated levels of aluminum in the brains of Alzheimers patients so they naturally linked it to the disease. Now though, studies are showing that the Alzheimers may cause the elevated aluminum levels rather than the other way around, and that aluminum causes no harm to the body.
Also, I have a horrible cook top in my apartment and often find myself doing dishes requiring searing. I can get around this using cast iron which holds enough heat that I can finish searing before the thing noticeably cools down.
http://www.healthy.net/asp/templates/article.asp?pagetype=article&id=1958
With use of aluminum pots and pans and aluminum foil, some aluminum leaches into food, especially with acid foods such as tomatoes or rhubarb. Cooking with fluoridated water in aluminum cookware increases the aluminum in the water and the food; still, the amounts we obtain in this manner are small in comparison with those from additives. Aluminum salts used in antiperspirants are not a major contaminant either, unless these products are overused. (Aerosol sprays, however, should be avoided for environmental toxicity reasons.) Antacids containing aluminum hydroxide can be a big source if they are taken regularly or abused, as antacids sometimes are. Some children's aspirins have been found to contain aluminum as well.
http://www.doctoryourself.com/alzheimer.html
A single aluminum coffee-pot was shown to have invisibly added over 1600 mcg aluminum per liter of water. This is 3,200% over the World Health Organizations set goal of 50 mcg per liter. Aluminum is known to build up in the bodily tissues of persons with Alzheimers disease, Parkinsons disease, and amyotrophic lateral sclerosis. Aluminum is a known neurotoxin. Aluminum is also a component of so- called silver amalgam dental fillings. Composite (white) fillings do not contain aluminum (or mercury, for that matter.) Most baking powder contains aluminum. Rumford brand baking powder does not, however. Neither does baking soda, which is a different substance entirely.
FACT: More than half of nursing home beds are occupied by AD [Alzheimers Disease] patients.
FACT: Alzheimers disease is the Number 4 Killer of Americans, causing over 100,000 deaths each year in the USA alone.
In new tests conducted by a university food safety professor, a generic non-stick frying pan preheated on a conventional, electric stovetop burner reached 736°F in three minutes and 20 seconds, with temperatures still rising when the tests were terminated. A Teflon pan reached 721°F in just five minutes under the same test conditions (See Figure 1), as measured by a commercially available infrared thermometer. DuPont studies show that the Teflon offgases toxic particulates at 446°F. At 680°F Teflon pans release at least six toxic gases, including two carcinogens, two global pollutants, and MFA, a chemical lethal to humans at low doses. At temperatures that DuPont scientists claim are reached on stovetop drip pans (1000°F), non-stick coatings break down to a chemical warfare agent known as PFIB, and a chemical analog of the WWII nerve gas phosgene.
http://ww3.komotv.com/Global/story.asp?S=3615478
A $5 billion class-action lawsuit is being filed against DuPont for failing to warn consumers of the dangers of an ingredient allegedly contained in Teflon, lawyers said Tuesday.
Two Florida law firms told the Associated Press they were filing the federal suit on behalf of 14 people in eight states who bought cookware coated with non-stick Teflon. It reportedly is made with a chemical called perfluorooctanoic acid (PFOA), which earlier this month the U.S. Environmental Protection agency said is "likely" to cause cancer in people, the AP said.
The plaintiffs contend DuPont has known for more than 20 years that the product caused cancer in lab animals, according to the AP. A company spokesman said federal tests "show that nonstick coatings used for cookware sold under the Teflon brand, do not contain any PFOA." Spokesman Cliff Webb added that DuPont would "vigorously defend itself against the allegations."
So, does nikel leach into your food from cheaper stainless steel pans and does aluminum leach in from Calphalon? Why did the water taste so much worse out of the Calphalon and what can it mean other than that we tasted the metals from the Calphalon pot?
EDITORIALS: THE SATURDAY PAGE THE EPA FOLLIES
Sticking up for health
WHEN IT COMES TO TEFLON, the Environmental Protection Agency is living up to its name. By calling this week for the reduction and eventual elimination of a potentially dangerous chemical used to make Teflon, the agency has shown it is willing to protect global wildlife and human health.
Unlike Teflon presidents or Teflon sports figures, Teflon itself has sticky health issues that refuse to slide away. A troubling compound used to make the slick stuff, called PFOA, is found in 95% of Americans and has been detected around the world, even finding its way into polar bears in Greenland, Alaska and Canada.
It's not clear how this chemical finds its way into the environment; it's removed from Teflon and other products during the manufacturing process. But PFOA is practically ubiquitous, used to make waterproof clothing, phone cables, building materials and more. In animal tests, it has been found to cause birth defects and has been linked to cancer and immune suppression, among other health problems. It stays in the human body for years and is passed on to a fetus during pregnancy.
Last year, the EPA went aggressively after DuPont Co., saying the chemical powerhouse that pioneered the use of PFOA had been hiding the substance's health risks for close to 25 years and had failed to report that PFOA had seeped into residential water supplies in Ohio and West Virginia. DuPont agreed last month to pay the largest administrative fine in the EPA's history: $16.5 million.
It would take years for the federal agency to ban PFOA; instead, in a rare move for the EPA under any administration, it called for preventing its release into the environment. Releases would be cut by 95% by 2010 and eliminated by 2015, it appears the eight major companies that use the chemical are agreeing to go along.
Before anyone slaps a halo on the EPA's head, it's worth noting that the agency is comfortably following a parade that was headed in this direction. DuPont has agreed to pay a settlement of up to $342 million in the water-contamination case — incentive enough to control its release. The company reports that it already has cut emissions of PFOA over the last few years by 94%.
Still, EPA officials made the right move. It's good to see the agency back in action; here's hoping it lasts.
http://www.costco.com/Browse/Product.aspx?Prodid=10048379&whse=BC&top...s=1
I might prefer the tempered glass lids over stainless to view the cooking action, but my web research hasn't unearthed anything close in either 13-pc sets or copper bonding to this set @ 199.99. Closest ones are Emeril or Cuisinart on Amazon, both of which have less or zero copper and fewer pieces for the same price. No idea of the precise thickness on the stainless-alu-copper-alu-stainless layers, but I can guess no less than industry standard from the physical heft of in-store demo pieces and general online discussions.
Does anyone here have experience or an opinion on the value and quality of this as a jack-of-all trades starter set? I've generally had very good results with Kirkland private label products, save the reduced prestige from the brands they compete with head-to-head, but I could care less about the brand if the goods are legit and cost 50% less than the equivalent.
Thanks in advance for any thoughts.
Mel
Anodized aluminum and teflon are two different things.
Anodized Aluminum is a type of aluminum that has been treated to provide an thin but extremely hard aluminum hydrate layer. This layer is non-reactive, so it does not have the downsides of plain aluminum (which may react with acidic foods changing their color and taste). When cooking, treat anodized aluminum pans as if it were a (black) stainless steel clad aluminum pan. It is possible to put a nonstick layer (such as Teflon) onto an anodized aluminum pan, but I don't really see the point - you'd be covering up the ultra-hard layer that was put on.
Think of Teflon as the clear coat of your car's paint job. It makes the surface nice and smooth and slippery so it's easy to clean off. They can put a coating of Teflon onto basically any cookware material that you can scuff up in manufacturing - stainless steel, aluminum, and sometimes even titanium (for lightweight camping).
Calphalon has a newer line of pans that they call infused anodized aluminum that is supposedly nonstick without the use of Teflon. For some, it seems to work magically well - sticking to produce fond when they want it to and releasing at just the right time. For others, it's just a pain to work with. Your milage may vary.
So far, my favorite non-stick (Teflon) pans are made by Scanpan. (I use this 9-1/2 in. fry pan whenever I make eggs and other "sticky" foods. I use stainless clad aluminum for just about everything else.)
Do you have an opinion on "waterless" cookware. Kuhn Rikon and Lifetime carry this stuff. It is VERY expensive, but if you ever sat through a Lifetime Song and Dance at a home show, you would think you are actually killing your family if you don't buy this stuff.
Please advise if you or anyone out there is familiar with this stuff.
Thanks much.
Can someone explain to me--in lay terms (I didn't do so well in science in school!)--why teflon pans don't heat up on induction burners? Everytime I put a teflon pan on an induction burner, the burner turns off. It's a great mystery to me!
Can someone explain to me--in lay terms (I didn't do so well in science in school!)--why teflon pans don't heat up on induction burners? Everytime I put a teflon pan on an induction burner, the burner turns off. It's a great mystery to me!
Your teflon pan is most likely made out of aluminum. Induction cooktops work only on cookware that is affected by a magenetic field. An easy test to see if you cookware will work on an induction cooktop is to take a magnet (like a refrigerator magnet) and see if it will stick to the bottom. High quality stainless steel clad aluminum (such as All-Clad but not Farberware or Calphalon's new line of cladware) and cast iron are your most likely candidates.
. . . .. Aluminum is also a component of so- called silver amalgam dental fillings. Composite (white) fillings do not contain aluminum (or mercury, for that matter.) . . . .
Well, what can I say. I am a Prosthodontist. I am an anal, and ultra-compulsive dental bioengineer. A Prosthodontist is a dental specialist who has spent three years in an accredited program after four years of dental school studying the restoration and replacement of teeth, the construction of fixed and removable tooth and implant supported prostheses, cosmetic dentistry, and is an expert in dental biomaterials. The earliest silver amalgam was made in the early 1800's, but the most similar predecessor of modern silver amalgam was invented in 1895 by GV Black--and this was the basis for all modern amalgams. The most modern/currently used silver amalgams, produced by numerous manufacturers (let's say after the mid 1970's) is a eutectic alloy of silver, tin, copper, palladium, indium and mercury. A eutectic alloy is a mixture of substances in fixed proportions that melts and solidifies at a single temperature that is lower than the melting points of the separate constituents, or of any other mixture of them. To my very best knowledge, THERE HAS NEVER BEEN ANY ALUMINUM IN ANY DENTAL/SILVER AMALGAM. If however you have real information, such as a product insert (always supplied) which contains the exact ingredients accompanying the specific brand of amalgam you are referring to, NOT something cut and pasted from some ridiculous website, then I am interested in the reference. If not, what I say here is the total fact on that matter. Can you tell this is a sore point with me?
Next, while resin composite fillings (tooth colored composites) do not have mercury which many people are so unnecessarily concerned about especially since the advent of high copper and palladium containing alloys in the mid 1970's, let me be the first to inform the wholistic lovers of composites that the epoxide resin component of the composite contains compounds very similar to/congeners of estrogens. About 10-15 years ago this was brought to light, but no one wanted to hear this because everyone wanted tooth colored fillings. SO . . . all you people who want really fine dentistry, you have two main choices--gold or porcelain. The finest material to use in small cavities would be compacted gold foil, followed by cast gold inlays for most situations and then gold or porcelain fused to gold crowns, which are the benchmark ("gold standard") by which all other materials are judged. While porcelain and other ceramic tooth colored materials look natural, they tend to wear down the opposing teeth faster than another tooth or gold chewing against it. Furthermore, all-ceramic/non-metallic restorations generally need to be cemented/"bonded" by a resin composite cement or else they break. So, you can't totally avoid the composite here either, and it doesn't seem to be a problem either, although you can minimize it's surface exposure by using the material as a cement, rather than the bulk restorative material. Now that you know that nothing is perfect, you can sit down and try to decide where the real dangers (if any) really are and what you would like in your own mouth. You will get more mercury from eating large fish--at the top of the food chain, because heavy metals tend to be cumulative in tissues--than from silver amalgam fillings.
I'm sorry about the tirade, but there is so much bad information out there that I am bombarded with on a daily basis that I had to set this part straight, hopefully falling on intelligent ears.
As far as the "purity" of the special cookware mentioned by a guest on Jan 24. 2006, that is "purer" than 18/10 in cheaper pots and pans that a guest poster was concerned would leach nickel, let me say that cookware and flatware comes in 18/8, 18/10 and rarely 18/12--the best. The first number is the amount of chromium that is contained in the stainless, i.e., 18 is 18% chromium. The second number is the amount of nickel, i.e., 8 stands for 8% nickel. So 18/8 means that this stainless steel contains 18% chromium and 8% nickel. 18/10 is 18% chromium and 10% nickel. The higher the numbers the more corrosion resistant the material. 18/0 is a misleading designation. Both 18/8 and 18/10 contain nickel and are part of the grade family "300 series" stainless. 18/0 means that there is 18% chromium but zero nickel. When there is no nickel the stainless grade family is the "400 series". 400 series are not as corrosion resistant as the 300 series and are magnetic, where the 300 series are non-magnetic. Therefore the more expensive/better/shinier stainless steel alloys have MORE nickel. Those alloys with more nickel are more corrosion resistant and have a brighter shine/luster. Regarding the term "purity", if the cookware the guy was selling was 100.000% pure lead, it would be very pure, but I wouldn't want to eat from it. Using the word "purity" is VERY misleading.
Regarding Micheal Chu's comments on June 7, 2006 on induction cookware, as he stated, the cookware must be affected by magnetic fields to heat up. Therefore, they (the All-Clad and others) must be 400 series stainless steels OR be 300 series and have a different magnetically inductive meterial in the base of their cookware. Maybe that information is a proprietary secret. It's a pity you can't use tinned copper--generally recognized as the best cookware on an induction stovetop.
The aluminum in cookware is another discussion, and I don't want to get into here, but I will say aluminum as a cause of Alzheimer's Disease is very controversial and definitely NOT proven. Some researchers believe the aluminum is a CAUSE, while others feel the accumulation of aluminum is the RESULT of the disease. Since we are on the topic of metals and Alzheimer's, zinc is another metal being looked at as a cause. Try doing some research in the National Library of Medicine and the National Institutes of Health. The verdict is definitely NOT IN on aluminum, in fact there are many other suspected causes which have more evidence than aluminum.
Since concern about aluminum in antacids was mentioned in another post, here is a list of aluminum containing antacids:
Di-Gel liquid
Gaviscon tablets
Gelusil liquid
Gelusil tablets
Extra strength Maalox
Mylanta & Mylanta Double Strength liquid & tablets
Tempo Soft Antacid
These antacids are Aluminum-free antacids:
Alka-Seltzer
Alka-Mints
Di-Gel tablets
Maalox caplets
Mylanta gelcaps
Rolaids tablets
Titralac
Tums E-X
Thank you, and have a nice day! :)
The statement by nurmich (April 21, 2006) is not correct. Teflon will be here.
Teflon PTFE and e-PTFE ---- expanded PTFE (Gore-Tex) saves lives. The cooking counterpart is OK too. e-PTFE (Gore-Tex) is used in surgery in several medical fields. There are many different configurations of Gore-Tex used in surgery. Google it. It is notably used in cardiovascular surgery for heart and great vessel grafts and repairs and in oral surgery bone grafting procedures. What is to be eliminated is the Perfluorooctanoic Acid (PFOA) from factory emissions and finished products by 2015.
http://www.epa.gov/oppt/pfoa/
http://www.epa.gov/opptintr/pfoa/pubs/pfoastewardship.htm
http://www.epa.gov/opptintr/pfoa/pubs/pfoarisk.htm
If you don't like Teflon, you can always use SwissDiamond non-stick cookware. It is not a teflon product. It is VERY good but fairly expensive. I like it a lot.
I do not want to use ' Teflon ' - nor is the word used anywhere on the labeling.
Iwould like to know what is used on these sheets and why the Canadian Government allows the product to be sold without clear definition.
Can anyone provide knowledge on this substance?
Thanks :angry:
Hi:
There might be some minor difference in performance that could be detected if Julia Child rose from the dead and cooked with them side-by-side, but there is not that much difference in most quality tri-ply cookware. Where there IS a difference is in Cuisinart's approach to marketing. They often change the design and specs of their cookware to update the fashion appeal of their lines, and when they do, you're not able to get the former version for very long. This makes a warranty less meaningful than most of us would like it to be. Let's say that five years from now, you have trouble with a Cuisinart skillet. You got a "limited lifetime warranty" with it, so you're fine, right? Well, maybe not, because if Cuisinart has discontinued your cookware in favour of something else that has become fashionable, there may be no replacement stock with which to honour your warranty claim. You'll get an offer to replace with a "comparable" piece, or you'll get some other offer. What you won't get is a new piece just like the old one. Cuisinarts is in the process of phasing out its "Everyday" cookware, the classic copper-stainless sandwich construction, probably due to the rising cost of copper. All Cuisinart lines now feature aluminium in the sandwich. If you valued the copper sandwich enough to pay well for it, would you be happy with an aluminium-sandwich piece to replace it under warranty?
All-Clad is also not a very consumer-friendly company, from what I've heard from friends still in the biz (I was in the kitchenwares business for six years), and the stuff costs what a mortgage payment used to. If you would like to save a lot of money over the price of All-Clad, and get essentially the same quality and performance, I would suggest you take a look at Tramontina's Tri-Ply line. It's so close to All-Clad you can hardly tell the difference in some pieces. Tramontina is a Brazilian company that now makes cookware in the United States (they bought the old Mirro plant in Manitowoc, WI for the purpose- nice to see a foreign company bring jobs HERE for a change, eh?), and in China.
If you want to see some Tramontina Tri-Ply, with pricing, go to: http://www.kitchenfantasy.com/shopping_cart/sspots.html You should be aware that Tramontina, like a lot of other companies, makes several lines of cookware, with Tri-Ply being their highest quality. Be absolutely certain that any Tramontina you buy is Tri-Ply, not one of their lesser lines like Sterling. Be especially careful on eBay; some ignorant sellers (I'm giving them the benefit of the doubt here) describe all Tramontina as tr-ply, because of the three-layer sandwich on the bottom of the cheaper lines. ONLY the Tri-Ply line has the same straight-gauge construction as All-Clad, with a layer of aluminium between two layers of stainless, that goes all the way up the sides of every piece.
Good luck!
For sauteeing and especially for delicate sauces that require control, nothing is better than copper, for its fast response.
But fast response is a problem for other things. If you want to deeply brown or blacken a large piece of meat, you want heat retention. The best material is probably heavy cast iron, with its high density and high specific heat. The pan will maintain high temperatures even after the room temperature food is thrown onto it. Heavy, slow response materials like enameled cast iron also work especially well for slow simmering (I don't fully understand the physics behind this, but a couple of big batches of stew or soup in a dutch oven will sell you on these qualities).
A minor issue with copper is its poor ability to keep food warm. That fast response means your sauce will cool rapidly as soon as the fire goes out. If you use copper sauce pans (lucky you if you do) it's worthwhile to adopt professional techniques like a bain marie (water bath) to hold the food at temperature, if you can't serve immediately.
Heavy aluminum cookware comes somewhere in the middle and is a good general purpose solution.
Surface material is also worth strong consideration. Like most cooks, I'm starting to prefer stainless steel interiors for most pans. It does almost everything well, and its bright color makes it easy to judge the state of carmelization (technically the Maillard reaction) of pan juices. Very important in any kind of sauteeing and roasting. Clad metal comes to the resucue: 18-10 stainless lined copper or aluminum.
I like my anodized aluminum pans, too, but the surface is definitely harder to use, and ultimately doesn't hold up as well.
If you cook a lot of eggs, you should have at least one non-stick pan. Get a cheap one. They don't stay nonstick very long. Even the ones warranted forever are only warranted to keep the surface intact ... they say nothing about how well it will actually work.
Naturally nonstick surfaces like seasoned spun steel or cast iron work great for things like fish, but beware of using for omlettes ... your breakfast will taste like fish, fried dumplings, or whatever else you cooked in there last night.
If you choose clad or laminated aluminum, keep in mind that pans vary greatly in overall thickness and thermal mass. Pans that look the same will have very different cooking characteristics. There isn't always a right answer as to what's better ... what you cook, how you cook, and what you're used to will play into this. Best to borrow some pans before investing.
And don't buy a set. False economy. Get what you need, in the material you need, one at a time.
Some of these pans may be decent, but if you want the benefits of professional copper cookware, you need the real deal: 2.5+ mm thickness, usually clad with 18-10 stainless, almost always with a cast iron or stainless steel handle.
The more traditional pans are tin-lined. These offer some advantages (slightly faster response, and they are re-tinable) but many disadvantages (fragile surface, and low melting point of tin makes many kinds of cooking impossible).
The brands I can vouch for are Mauvielle, Bourgeat, and Falk.
Copper prices are ridiculously high right now.
I was wondering if you could recommend a safe but low maintenance pot (~ 10 quarts) for making beef stews, soups, and other slow-cooking foods?
Your advice is greatly appreciated (I've been pulling my hair out trying to decide)!
I have cooked waterless on 18/10 and t304 and t316L its all about cooking on low heat and haveing atlest a 5 ply cookware.
The thing I don't like most about teflon is that if you cook at high temps with teflon, it loses it's non stick properties. For instance, if you cook bacon in a teflon pan, over a very short period of time, that pan will loose its non-stick properties. Teflon is not a long term solution for cook ware. As for multi-clad cook ware, I was a chef for many years and cooked on pans that heated from the bottom. I am not sure I want a pan where the sides are as hot as the bottom. I need places in the pan that are cooler so I can bring the food to the heat and when its done, rotate it up to the top and bring more food to the heat. I could have bought another brand of multi-clad cookware that was significanlty less expensive, but I wanted cookware that I was used to cooking on. My 17 piece set cost $249 at Chef's Catalog. If I would have purchased them separately it would have cost more than $700. So I defeinitly recommend buying a set rather than one at a time. Also, make sure the set has what you need. The All Clad set, 9 pieces for $800, has a 3 1/2 qt saute pan. This is a very small pan. Also, things like a vegatable steamer insert typiclly get very little use unlike, say, a pasta cooking insert. I would also not get a copper set because copper is so soft that the pan dents and will not make contact with electric cooking surfaces.
A friend of mine has a Calfalon anodized aluminum set and he has sent several pans back for warrantee coverage because that pans failed over time. I don't want to be shipping cookware every few months.
Finally, I bought a set of Calfalon cookware (about $3000 worth) and took it back because everthing I cooked in it stuck to it. I could not get some of it off when I retuned the set. Also the care instructions for the Calfalon are draconian. Apparently normal use of this cookeware leads to performance failures.
In summary, the Cusinart Chef's Proffesional cookware performs very well, cleans well. You can use oven cleaner on it if you have a major burn, unlike aluminum. I have had trouble with Calfalon as well as some of my friends and I don't think I would like the multi-clad cookware because it changes the way I am used to cooking.
Thanks for listening.
The thing I don't like most about teflon is that if you cook at high temps with teflon, it loses it's non stick properties. For instance, if you cook bacon in a teflon pan, over a very short period of time, that pan will loose its non-stick properties. Teflon is not a long term solution for cook ware. A friend of mine has a Calfalon anodized aluminum set and he has sent several pans back for warrantee coverage because that pans failed over time. I don't want to be shipping cookware every few months.
Finally, I bought a set of Calfalon cookware (about $3000 worth) and took it back because everthing I cooked in it stuck to it. I could not get some of it off when I retuned the set. Also the care instructions for the Calfalon are draconian. Apparently normal use of this cookeware leads to performance failures.
I have always felt (and you can see other posts of mine here) that Calphalon is a well advertized, high priced, low quality rip-off.
The likely reason you have had sticking with teflon is because you probably have "seasoned" the grease on it which is preventing the teflon from acting as a releasing agent. There might be other reasons, but try really cleaning the pot VERY well and see if the problem goes away.
I was wondering if you know the exact material used for wok pans. I know that there are many variations for its manufacture, but do you know what exact type of cast iron? or carbon steel, or aluminium?
thanks
Mish
Traditional Chinese woks are made of thin cast iron. As to the type of cast iron, I don't know - most likely some form of grey cast iron. You can give The Wok Shop a call or send them a message asking if they knwo the specific type of material used in their cast iron woks. Also, during the last several decades, carbon steel has become more popular as a material for woks both in China and in the U.S. The specific type of cast iron or carbon steel is dependant on the manufacturer.
We have a large fish kettle that has a generous coating of what appears to be zinc inside and out. We used it for the first time last night over our stove's gas burner to poach a trout. The poaching water had stock, wine and onions, and the resulting poached sea trout was delicious. We boiled down the poaching water ain the kettle to a seriously good-smelling stock.
On emptying the stock the next morning we were horrified to find that the zinc lining on the inside had been melted away above the burner jets and there were small blobs of zinc loose in the bottom of the kettle.
We ditched the stock. We don't appear to have any of the usual symptoms of zinc poisoning.
I have been trying to reproduce the melting effect this morning. Over an hour or two of boiling, there is no melting but there seem to be some new small loose blobs of zinc (qty 3 @ 1mmx2mm) forming again, indicating a dissolution/precipitation process. Why would this be happening? Why wouldn't any zinc in solution plate back out onto a cooler part of the vessel wall? Isn't zinc a safe coating for cooking vessels?
A Canadian government food health site warns against cooking or storing in zinc any foods or liquids with a pH below 4.5. I suppose its possible that the poaching water was made acid by its constituents, and more so by the reduction, but we have no means of measuring pH.
Our water comes from a series of rainwater tanks - a main concrete tank with some other plastic and galvanised tanks. We also have a galvanised roof and tend to have leaf litter in the gutters. I imagine that the leaf litter may acidify the water, but the concrete tank walls should neutralise that.
Do we need to be concerned about zinc migrating off the kettle into the fish? At what point would the vessel stop dissolving zinc into the hot water? Would this happen with perfectly neutral water?
Thanks,
Matt and Anna
If you look at any blog site in any topic, everything has been discussed before someplace, but times change, technology moves on, different people have more varied viewpoints, and sometimes the answers to questions change as more data becomes available. In some topics, we have links to other references, giving them credit where it is due. So, in view of the fact that we are not plagiarizing other blogs, no credit is due anyone, as we all have original writings on the topics we discuss.
Tell me if i horribly screwed up somewhere, because these numbers seem a little one sided to me.
For sauteeing... ...nothing is better than copper, for its fast response.
Really? I don't have a saute pan, but i assumed aluminum was better, since it's significantly lighter. So is copper so much better that the huge weight is worth it?
I also want to thank the Prosthodontist for his informative comments. I still prefer to not take any chances with aluminum since any future test results won't do me any good after the fact. I don't find the use of aluminum any thing I will miss. My original question was to find out rather Nordicware was made with Teflon. It appears it is and so I search for a grill to put on top of the stove that doesn't have such things on it.
Does one exist?
if you're willing to pay for good pans, take a look at the traditional copper styles - now available lined with stainless. I have some of the 3mm Bourgeat and they are superb.
thermal conductivity (BTU/(hr-ft-'F) of
aluminum = 136
copper = 231
silver (the highest) = 248
stainless (304) = 8
Fold a dish towel into a pad and hold the handle with that. Works great and still lets you shove the cast iron into the oven for finishing.
>>What works best with flame cooking?
there's no one single absolute answer to the question.
much depends on how you cook and the extend of your own personal "demands"
huh? you said?
my dear mother does not know the meaning of "sear" and survived just fine for decades with her RevereWare
however if you tend to do high heat type stuff, you may find RevereWare lacking "technique support"
on the other hand, you can boil / steam green beans in anything.
I have a Farberware stainless pot with clad bottom that is de-laminating - severely - to the point it's headed for the trash.
so "clad" cookware is also not the end of all things.
non-stick:
yup - you've got a problem there. gas can overheat a non-stick pan to 400-500'F in very short order - and convert a non-stick to a super-stick in just a few seconds - and kill your pet bird just as quick.
that said, I use non-stick all the time on a larger diameter high BTU nat gas burner - do NOT go for the "preheat the pan" thing....
works fine for sauting mushrooms / high moisture content schufft you need in short order.
trying to fry an egg in a thin stainless - copper clad or other - over gas is an interesting exercise.
not recommended when cooking for company, but educational - and very much in tune for the times - instant graduation from that idea . . . .
cast iron - great stuff.
handles: I actually have one square frying pan with a screw in wooden handle.
one point of cast iron is cooktop-to-oven.
the high end 'other' brands do not have "plastic" knobs or handles that will stand up to high oven temps over time.
so, if you do cooktop-to-oven, get some potholders & trivets.
if you watch the cooking shows, it's most interesting to observe the background of real restaurants - seen now and then, depending on chef / show - real locations that prepare food - not film stages.
I can categorize two "real" scenes:
- a not high end place. run of the mill aluminum saute/fry pans - all warped and grungy.
- the higher end places - racks and racks of grungy copper pots/fryers/sautes/sauce pans hanging about.
a third scene is stuff like Julia Childs' home kitchen - walls of hanging copper pots & molds - all brightly shined. never used; decoration.
bottom line:
you need pots for boiling / steaming - glass, stainless, alum - take your pick.
you need cookware for slower evenly distributed heating - omelets to (non-burnt) sauces / stews.
thick bottomed alum / stainless / clad / copper
you need cookware for the sear at megamillion degrees followed by oven finishing: cast iron or copper.
how many of each, what sizes / shapes, will be a concerted compromise of what & how your personal preferences & habits extrapolate.
What you have noticed about the burning of food on thew sides of pans is common on gas stoves because just about every one on the market has a ring shaped burner, which allows the flame to basically go around the sides of the pan without actually heating the bottom. You should try a smaller diameter burner. If you can afford it, getting a REAL restaurant stove or a Thermador professional home stove, both of which, have star shaped burners, which heat the entire bottom of the pan, is also a good thing to do. B)
yup, won't get much info on that site. marketing pizzazz - no meaningful detail, no meaningful specifics.
Regal / West Bend is an established old line company, but they don't jump to mind when someone mentions "qualiity stuff"
there are several "exclusive" brands being marketed by the parent company with all kinds of fantastic claims. many of them are MLM schemes - probably cost 60-100 times its value.
not everyone is impressed:
http://www.nicolekruse.com/company_201.html?utm_source=Google&utm_medium=PPC&utm_campaign=lifetime%2Bcookware
http://www.tian.cc/2005/12/overpriced-cookware-by-classica-and.html
As Dilbert said the type of pan you use depends on what you are cooking in it.
the best approach would be to get the exact manufacturer's part number - hopefully some information is stamped somewhere on the pots - and contact them at wearever.com
to my knowledge, and I am not a metallurgist, nickel is not a common component of aluminum alloys - nickel is almost always present in stainless steel.
when nickel atoms/ions/compounds can leech out of stainless steel is a question for an expert - which I am not.
Nickel can also leach out of coins. What happens when the child handles coins like nickels, dimes and quarters?
Has the child had nickel allergy confirmed by an allergist, or is it just a guess?
I found this website, in a search to help me with these questions.
Thanks for all input.
Gloria
I found this website, in a search to help me with these questions.
Thanks for all input.
Gloria
The water tastes different from different kettles because of the differences in reactivity of the metal the pots are made of. Some stainless pots may have solders or fluxes that remain that can affect the taste of the water. A plain stainless steel pot wil probably taste best because stainless is least reactive and will have least interaction with the water as it heats and boils. My feeling is a an all stainless pot, not even one with a copper bottom is best. I have a Krups electric kettle and it makes fast, clean tasting boiled water.
Also make sure the clean out the pot occasionally even though only water is placed in it because in some areas, lime may deposit on the walls of the kettle.
Swiss Diamond contains PTFE, just not Teflon (TM) PTFE. The Swiss Diamond FAQ states this very clearly. If you believe that you are avoiding some terrible fate by using Swiss Diamond cookware instead of Teflon cookware, you are misguided. PTFE, regardless of brand name, will degrade and release small quantities of mildly toxic fumes -- not particularly harmful to humans, but they might kill a pet bird.
So, don't believe that Swiss Diamond is somehow safer than Teflon. However, both, when treated well, pose absolutely no health risks. Smoking oil on the stove is a far greater cancer risk than over-heated teflon. Buy your cookware based on how well it works for you, not groundless health claims. And get a range hood for your stove if you're really worried.
Thanks,
Gail
I tried the link on multiple computers and couldn't find any problems.
Essential Cookware - http://thebestcookware.blogspot.com/2008/05/essential-cookware-for-your-kitch...tml
How to choose frying pans - http://www.salamandercookshop.com/index.php?main_page=page&id=11&chapter=0
We have been proponents of the benefits of stainless steel cookware in terms of sustainability for years. I personally wanted to call to your attention what the Specialty Steel Industry of North America (SSINA) says about the benefits of stainless steel in terms of sustainability.
We've paraphrased their comments here:
http://www.justsmartliving.com/blog/2008_10_01_archive.html
And their original article can be found here:
http://www.ssina.com/overview/features.html
I like the carbon steel pans for certain things, great for throwing in the oven, just not quite what i was expecting.
http://www.lodgemfg.com/use-care-seasoned-cast-iron.asp
indeed you should research the brand and its specifications very carefully.
a number of companies are marketing "titanium" cookware. aside from camping gear (which is just plain metal, lightweight) all of the brands I have checked use a sputter coating of titanium, which creates a sandpaper like surface, and the "holes" between the "grains" is filled in with PTFE - the generic name for Teflon (a DuPont trademark.)
most of the marketing hype will say "does not contain Teflon" - well, if I fill up my car gas tank with gasoline, not Exxon, what's the diff?
many marketing sites simply state its a proprietary thing - they don't say if it does, or if it doesn't.
also note the warranty info on many sites: if you cook too hot, cooking fats will coke on the pan and void the warranty.
note also the "safe to 500 degrees"- titanium will go a whole lot hotter than that, what's holding them back?
interesting link: http://www.naturalnews.com/021059.html
SwissDiamond now states:
Do Swiss Diamond products contain “PTFE”?
YES! PTFE is the component that gives non-stick properties to the surface of the cookware and many other consumers’ products. Our patented inherent slippery coating is reinforced with Diamond Crystals which are amalgamated into a nano-composite (mixture of extremely thin particles). Thus it requires lower quantity of PTFE, much lower than most of other non-stick products.
at the basis of all the marketing hype and misinformation is the simple fact that PTFE is - so far as I have ever seen documented - the only durable "non-stick" compound in existence.
various "green pans" claim a silica based technology, which apparently does not last very long, judging by the consumer reviews.
see: http://dannyseo.typepad.com/my_weblog/2007/10/green-pan-updat.html
that thread also points out the need to be very aware of the site you are reading - as stated there, tv sales channels do not allow negative posts to remain - only the rave reviews.
As soon as I cooked anything in these pans, an unsightly oxidation appeared where the food had been. It cannot be removed and is now part of the pans.
Please, engineers, let me know what's going on.
Thank you
according to their web site it's 18/10 stainless steel - and should not discolor, etc.
I've got stainless steel pans that go back two generations that are not discolored or oxidized in any manner.
I'd be tempted to contact them and ask what's up - do note that the warranty explicitly excludes "discoloration" - a bit of clarification with regard to "oxidation" would be helpful.
Does this occur to all your stainless steel pans or only the Cuisinart? Is it oxidation (rust) or a white substance that has cemented itself onto the base of the pan? When you say it cannot be removed - what have you tried? I suggest Barkeeper's Friend and a soft sponge. If Barkeeper's Friend does not remove it, then there is something wrong. We can try to narrow down what it is once we find out a little more info.
un-lined "plain" copper is is frequently used in confectionery work - candies / sugar stuff / sugar panned toasted nuts.
it does not normally impart a metallic taste in those uses - which makes me wonder if it is an alloy, perhaps not solid copper....?
if you search of "retin copper" you'll find a number of smiths that can do that.
The review of the metal properties is pretty spot on, but the point of carbon steel isn't thermal properties, it's surface properties. You have to use some type of fat with eggs (I used clarified butter), but the result is as close to non-stick as you can get with a surface that tolerates metal utensils and will last forever. Oh yeah, and this gourmet pan costs under $40.
A 2mm pan is heavy enough to have thermal properties that is not quite as good as a thicker cast iron, but pretty darn close. Yet it is lighter, slick and makes perfect eggs. There might be other pans that can perform just as well, but none that will last 3 lifetimes and only cost $30-40.
check out www.debuyer.com. www.chefscatalog.com carries a nice selection of de buyer pans, but they don't have the Force Blue line. I got my pan at World Market.
Also you never talked about the transmission of heat from the cooking material to the food. Does that just mirror thermal conductivity of the fire to the cooking material? In other words if I have two almost identical pans made of magic materials where the only difference is that pan A has a low thermal conductivity while pan B has a high thermal conductivity, what would happen differently if I heated both pans up to 300 degrees F and put a big steak on each?
But as you said
thermal diffusivity = thermal conductivity / (density and specific heat)
So if specific heat goes up then thermal diffusivity goes down. But specific heat is the ability to "hold energy." So then how can you say that a high thermal diffusivity means that a material can hold energy well?
In these idealized examples, two identical pans except one with low thermal conductivity (A) and one with high thermal conductivity (B):
A has a lower thermal diffusivity than B. Because heat capacitance is the same, both pans have the same amount of thermal energy to potentially contribute to the food, but A will cook slower than B. The steak will take longer to brown and cook through on A than B. The closest example I can come up with in the real world is a stainless steel (A) vs cast iron (B) pan, but it would have to be an exceptionally thick stainless steel pan or an exceptionally thin cast iron pan in order for the non-thermal conductivity variables to be somewhat similar. Usually, a cast iron pan has much more material volume (and thus total heat capacitance) than a stainless steel pan, so that makes this example a bit useless unless one can fine a sheet of stainless steel thick enough to match the heat capacitance of a cast iron pot...
In the second scenario (differing heat capacities) - this is actually one where a real world example could come into play. For the same material - let's do stainless steel - we have pans of different thickness. Thermal diffusivity of the pans are identical since the material has not changed, but heat capacity is higher on the thick pan because it has a larger volume of material and heat capacity = specific heat * specific gravity * volume of material. What happens when you stick a big steak on the thin pan and the thick pan is that cooking occurs at the same rate (same thermal diffusivity), but the pan with less heat capacity will "run out of energy" sooner than the other pan. If no additional heat is applied to the system, then it will stop cooking before the thicker pan. Considering the situation that heat (the burner) is added to the system, complicates matters a bit, but the end result is somewhat similar. The thinner pan will initially cook the same as the thick pan, but hot spots will form sooner than the thick pan as it begins to run out the energy that was initially stored in the pan and heating comes mainly from conduction from the burner through the pan material. The thicker pan will eventually reach this point, but the process will be a little slower as there is more of a "buffer".
I hope that helped a little - I apologize for the lack of examples.
Yeah, that sentence is misleading. High thermal diffusivity means that the material can react to temperature changes rapidly (thus shedding or dishing out the energy stored in it rapidly), but it is heat capacity that determines how much it holds (like you said). It just so happens that in the small group of materials we examine in the article, they all have relatively high heat capacities. I'll think of a way to reword (or drop) that misleading statement.
So, if we talk about cooking spoons then its material should have low thermal conductivity and high specific heat capacity, right? So that the spoon doesn't get too heated up too easily during cooking.
So my question is: Which one of the following should be the properties of cooking utensils?
a) High specific heat and low conductivity.
b) Low specific heat and high conductivity.
We can't chose (a) because pans and pots should have high conductivity and low specific heat and we can't chose (b) because cooking utensils are also spoons so then cooking spoons shouldn't have high conductivity and low specific heat.
But I have to choose an answer, this is a very important college exam MCQ, what should I do?
I hope someone answers, I'll be waiting!
So my question is: Which one of the following should be the properties of cooking utensils?
a) High specific heat and low conductivity.
b) Low specific heat and high conductivity.
We can't chose (a) because pans and pots should have high conductivity and low specific heat and we can't chose (b) because cooking utensils are also spoons so then cooking spoons shouldn't have high conductivity and low specific heat.
I think this comes down to definition. What is meant by "cooking utensil"? I usually think of utensils as separate from cookware (pots/pans) so I'm thinking spatulas, spoons, stirring sticks, etc. But I understand there are parts of the country and world that call everything a cooking utensil. In that case, it's a stupid question since a spoon and a pot serves two completely different purposes - so how can the question be answered correctly?
Titanium's thermal properties are similar to stainless steel, but titanium is much stronger and most titanium cookware (designed for the camping and backpacking community) is extremely thin. In fact, when compared to equivalent aluminum camping cookware, the heating surface of the titanium pot is thin enough to produce similar heating times as that of aluminum (important when trying to conserve fuel). The big downside with such a thin pot? Hot spots. But, when camping heating food quickly in light cookware is more important than heating food evenly.
P.S. There is a little more to it than just a thin base - the thickness of the sides walls and rim of the pot can have an effect on water heating times because while the water is heating, the sides of the cookware are also heating. Aluminum will dissipate that heat faster than titanium, so boiling a completely full pot will probably be more advantageous to the aluminum cookware vs the titanium. (A half full pot will essentially have a tall ring of metal above the surface of the water acting as a radiator which will slow down the boil time. The efficiency of that radiator will depend on the design, mass, thickness, and material of the sides of the pot.)
You can definitely use these pans over electric or gas ranges. They will function like a normal high-quality pan.
These pans supposedly contain a magnetic layer that loses its magnetism once it reaches a mid to high temperature (around 485F). If it does do that, then the induction cooktop will stop working and the pan will begin to cool eventually regaining its magnetic properties which will allow the induction cooktop to heat the pan again (causing the pan to maintain temperature around 485F). Demeyere recommends using the pan on induction because they think this feature makes the pan superior to a standard pan and using the pan on any cooking surface except for induction makes it perform like a standard pan. There should be no safety/structural concern to using the pan on an electric cooktop aside for the inherent problems that would be present with any other pan.
Thanks.
anodizing does change the color and light/dark color absorb radiant heat differently - you would notice that effect.
I was thinking along the lines of the fact that anodizing makes the metal harder, and being harder it is perhaps more dense, and being more dense it would perhaps affect the time taken for thermal diffusion.
But like you say, maybe it wouldn't be noticeable.
e.g. the difference between say, all-clad MC2 and all-clad LTD2. (mind you, with all-clad I believe there's an actual thickness difference between these two lines as well).
I'm just trying to think like an engineer. :)
I haven't see manufacturers publish actual data for their pans. but the difference in the "pure" metals is so great that a little bit one way or the other is not going to make a lot of difference.
the "numbers" vary depending on units, but for:
Aluminum 136
304 stainless 8
with aluminum 17 times "more conductive" than stainless - the overriding effect is due to the basic metal - coatings & cladding aside.
I'm trying to decide what would be the most conductive and fastest material for the purpose of bringing water to a boil. Even if I can just shave off a minute or two in the boiling time, I would be interested in getting whatever is the fastest pot. I'm guessing copper would be the fastest, followed by straight gauge alumnum, followed by a disk bottom pot?
is there a way to make an educated guess beforehand? Or iare there so many variables involved that it's a matter of trial and error.
if you can afford a solid silver pots and pans, I'd like to introduce you to my daughter . . . .
>>I'm trying to decide what would be the most conductive and fastest material for the purpose of bringing water to a boil. Even if I can just shave off a minute or two in the boiling time, I would be interested in getting whatever is the fastest pot.
<<
after silver copper is the "fastest" material.
I'm not sure I can understand how shaving 60-120 seconds off the time to boil a specific volume of water on a 'same' burner could justify the cost difference of Walmat cheepie stainless to $multihundred copper - but heh, it's your kitchen.
copper has other 'features' - got a bunch of it, love it.
with 14 copper pots/pans at my disposal, if I'm heating water to cook/steam corn, beans, asparagus, cauliflower, broccoli, summer squash - well gosh most anything . . . I don't reach for the copper, I get out the old cheap thin 'worthless' stainless steel.
if I'm fixin' a roux, yeah you bet the copper pans are nbr 1 on the grab list.
Here's my completely unhelpful comment on thermal conductivity - diamond beats silver. Maybe in the future we'll have the technology to produce a diamond bottom cooking vessel.
Anyway, a more constructive comment would be that I find that using a thin stainless steel pot is the fastest way to transfer energy from a standard burner for the purposes of boiling water. We don't care about hot spots, just about transmitting heat into the pot as rapidly as possible. A thin, highly conductive metal would be better, but, in general, these highly conductive materials are made thicker because they need to hold more heat energy due to how rapidly they heat up/cool down.
If you are flexible with what burner you want to use (and not necessarily the one on your range), then getting a countertop induction cooktop and an induction ready pot will usually yield the fastest possible water boiling time because the electrical energy is converted to heat energy extremely efficiently. My $100 1800W induction cooktop generally boils water about 20% faster than my 17,000 btu gas burner. (My notes show an average time of 12.5 minutes to bring 3-qt of 40°F water in a 6 qt stock pot with lid on to a rolling boil with the induction cooktop while the gas burner in the same scenario took 15.67 minutes. Boiling times should be shorter in practice because most people don't start with refrigerated water, but I did for my tests because then I could keep the starting temperature constant through the testing.)
A friend in India has a 2500 Watt induction cooktop (They run 220 V) and that damn thing is so fast it's amazing!
I WANT ONE!
She can boils two cups of water at room temp (30 C°) in about 80 seconds, using a nothing more than a dented old stainless steel (well, it can't be all THAT stainless) pot.
I use this cookware especially because it does not have corrosion leaking into my food.
Thank you. :)
who makes a copper pan that is lined with silver?
Backpacking shops sell pots made from titanium metal. These are very light weight, because the metal has a low density, and is strong enough to be kept thin. They also tend to have a conventional nonstick coating. These are good for boiling water when ounces count in your backpacking gear, but that's about it.
There is something else that is labeled as titanium, but is really just a nonstick coating that includes a titanium ceramic. It's a sales pitch just like the diamonds of Swiss Diamond.
I looked up 316Ti. This is a stainless steel (like the common 18/10) with some added molybdenum to improve corrosion resistance in chloride environments (less salt pitting?), and a bit of titanium, which improves stability above 800C. So the Ti part adds nothing in home cooking applications.
I had purchased some Analon 5-ply SS/Al cookware as well as two Vollrath Tribute 3-ply fry pans. All the fry pans have warped notwithstanding that I use a grill surface thermometer to measure the temperature of the pans as they preheat. The 14" Vollrath (which is heavier gauge than the 12") held out the longest, and did not warp for about 2 years. The degree of warp is very slight, but on a glass top stove it is problematic.
Stainless steel with aluminium discs welded to the bottom have held out the best for me (so far). Wish I could go with gas, but that is not an option for me.
I have been using Rena Ware for that time, and still do. I have never met anyone who can replicate food done in a partial vacuum, using alternative methods.
Let alone the health benefits. The cookware looks as it did new, and to admit any bias, I sold it door to door for 2 years. It's carbon steel encased in Stainless. Fast, even,.....and works perfectly on the new induction tops.
Uses very little heat, reproduces colour, taste, and nutrition in cooked vegetables and fruits. Eliminates a large percentage of shrinkage in meat.
I now have a large sous vide system from Freshfood Solutions for doing the meat and fish proteins,......but the vegetables,......I've been doing that sous vide for 35 years!!!
Cheersk
oh the health benefits - only to the financial health of the MLM organization.
http://www.dailyfinance.com/2010/07/15/rena-ware-to-pay-600-000-for-making-false-health-claims/
Earlier this month, the Washington state-based company settled with the attorney general's office, agreeing to pay more than $600,000 in refunds and other fees as well as make changes to its business practices.
In its complaint, the attorney general said representatives for the company told consumers their competitors' cookware posed serious health risks. They told consumers that Rena Ware promoted good health and could cure diseases, claims that the AG says convinced consumers to allow sales reps into their homes.Consumers who agreed to buy the products were offered financing with interest rates as high as 21 percent, the complaint alleges. They also were't told they had a three-day period to cancel their order, which is guaranteed under California law for door-to-door sales.
http://dca.lacounty.gov/artRenaWareSettlement.htm
Rena Ware International, Inc., a company based in Redmond, Washington, agreed to pay more than $600,000 in fees and refunds to victims. Rena Ware must also have an independent overseer makes sure the company no longer uses false information or high-pressure sales tactics to lure customers.
http://www.consumeraffairs.com/news04/2010/07/ca_cookware.html
California Attorney General Edmund G. Brown Jr. today announced a settlement with Washington state-based Rena Ware International, Inc., which "made fraudulent and unethical claims" that its high-priced cookware could cure diseases such as diabetes and heart disease. The company agreed to pay more than $600,000 in refunds and other fees.
If you want to complain against Rena Ware or any other business, contact DCA:
Address: 500 W. Temple St. Room B-96 Los Angeles, CA 90012.
Phone: (800) 593-8222 or (213) 974-1452.
Is there anyone here that can shed some light on Silvinox??
[/b:33d024898e]
I want safe 18/10 or higher SS with nothing coming off into the food - either in the form of unhealthy non-sticks peeling off into our cooking OR any chemicals "leaching" into it. We gave up non-stick years ago after our parrot died & came to find out that those pans were the culprit...
Primarily I only want really SIMPLE cookware. I already use alot of carbon steel wok type things but I really want some gorgeous SS!! With copper core *only* no aluminum anything! I just hate aluminum! I know its great for conduction but for my cooking it doesn't help it ever and sometimes it actually hurts it! Mostly high heat Indian dishes so its LOTS of heavy duty spices and acids.
I'm pretty much focused in on Sitram Catering line bc its simple 18/10 SS with simple copper core. One Paella Pan from Mauviel (again copper & SS only)
BUT - here's where the twist comes in....
I am **IN LOVE** with a few pieces of straight-sided Atlantis from Demeyer - BUT - just not sure about that Silvinox stuff its treated with OR that TripleDuc 3 layer bottom on the Atlantis line. I don't like Aluminum so if thats in the 3layer bottom on Atlantis that might be a deal breaker; but I can't find any specs on WHAT that triple layer bottom IS exactly on the Atlantis line. Im gonna call them and find out - but in the end its that Silvinox & not understanding what it IS and IF its SAFE that freaks me out!!
well - that said - Silvinox is some sort of chemical treatment but I just can't find anything online that explains it in detail -OR- anyone out there talking about it on a safety level.
I've seen the ''process'' on youtube and i've read that its supposedly some sort of reverse-plating type thing that actually draws out some impurities and makes the SS harder and ''more pure'' - but STILL - what does that all add up to in high heat cooking? Is some sort of ''chemical'' there that can leach out??
I'm gonna ask them when I call about the bottoms all I can about this ''treatment'' - but still - I just wanted to run it by u guys here bc maybe there's someone out there with a Chemical background that knows something about it...
Thank you so much for ANY input u have on the subject!! I really appreciate it!
Abigail
[/i]
the two common "grades" of stainless are 18/10 and 18/8 - that's chromium (highly toxic) to nickle (highly toxic)
so you'll need to reach a decision about the toxic scare tactics as they apply to alloys.
similar applies to aluminum. the health hazard from aluminum cookware was dis-proven by multiple agencies half a century ago; your mileage is obviously on the variance.
>>copper not aluminum!
you'll be pleased to learn that for a given thickness, copper conducts heat twice as "better" than aluminum.
Silvinox is a surface treatment of non-described detail. last I looked stainless steel was the answer to that marketing hyperbole maiden's prayer.
what does that do for high heat cooking? nothing.
It's not a coating, and nothing is added to the surface. The stuff is expensive, but everything I've read says it's fantastically durable and easy to keep clean. I imagine one could eventually wear through the surface, given enough steel wool or scouring powder, but chromium is a very hard metal - the cookware should last pretty much forever given normal use and care.
Thermal conductivity: 2.0-2.3 W/m/K (0-400 deg C)
Specific heat: 815 J/kg*K (25 deg C) 1197 J/kg*K (500 deg C)
Density: 2500 kg/m3
Thermal diffusivity: 0.77 to 1.07 * 10-6 m2/s
@book{bolz1973crc,
title={CRC Handbook of Tables for Applied Engineering Science},
author={Bolz, R.E.},
isbn={9780849302527},
lccn={73166251},
series={Crc Handbook Series},
url={https://books.google.com.au/books?id=Xn8KbsgeFrwC},
year={1973},
publisher={Taylor \& Francis}
}
@book{guyer1999handbook,
title={Handbook of Applied Thermal Design},
author={Guyer, E.C.},
isbn={9781560328117},
lccn={98052353},
url={https://books.google.com.au/books?id=xVL3uGWew1UC},
year={1999},
publisher={Taylor \& Francis}
}
Copper cookware is enough heavy to sit on the burner securely but is also enough light to be lifted easily.
It has unmatched beauty as the richness and quality of copper cookware appeals visually.
actually, most are. metals contain traces to some to a lot of many many "elements"
the advertising is utter horse-droppings and you should go far far away from any company or brand with such "selling points"